
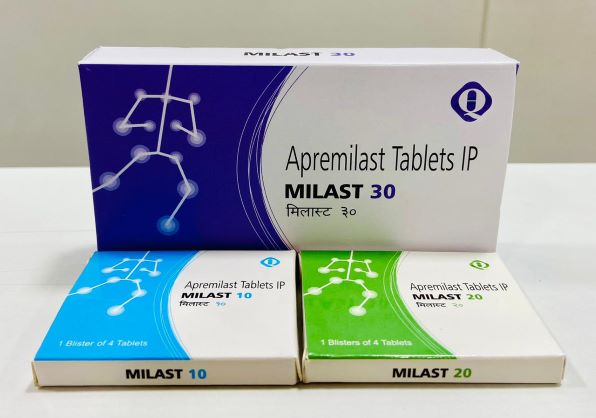
PRESENTATION:
Each Film Coated Tablet Contains Apremilast IP 10, 20 & 30 Mg Packing: 1*4 (10, 20 mg), 3*10 (30mg) Blister
CLINICAL PARTICULARS
Therapeutic Indications MILAST is indicated for:
The treatment of signs and symptoms of active psoriatic arthritis in adult patients.
The treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Dose and Method of Administration Dosage (Dose and Interval) Treatment with MILAST should be initiated by specialists experienced in the diagnosis and treatment of psoriasis or psoriatic arthritis. The recommended dose of MILAST is 30 mg twice daily taken orally approximately 12 hours apart. An initial titration schedule is required as shown below in Table 1. No re-titration is required after initial titration.
Table 1: Dose Titration Schedule
Day 1 Day2 Day 3 Day 4 Day 5 Day 6 & thereafter
AM AM PM AM PM AM PM AM PM AM PM
10 mg 10 mg 10 mg 10 mg 20 mg 20 mg 20 mg 20 mg 30 mg 30 mg 30 mg
Method of Administration
MILAST tablets should be swallowed whole, either with or without food. The tablets should not be crushed, split or chewed. If patients miss a dose, the next dose should be taken as soon as possible. If it is close to the time for their next dose, the missed dose should not be taken and the next dose should be taken at the regular time. Dosage Adjustment No dosage adjustment is necessary for elderly patients. Renal Impairment No dose adjustment is needed in patients with mild renal impairment. There are limited data on moderate renal impairment. MILAST should be dose reduced to 30 mg once daily in patients with severe renal impairment (creatinine clearance of less than 30 mL per minute estimated by the Cockroft-Gault equation). For initial dosage titration in this group, it is recommended that apremilast be titrated using only the AM schedule listed in Table 1 and the PM doses be skipped. Hepatic Impairment
Dose adjustment is not required in patients with hepatic impairment. The safety of MILAST was not evaluated in psoriatic arthritis (PsA) or psoriasis (PSOR) patients with hepatic impairment.
Monitoring Advice In the event of intolerable adverse events, interruption or discontinuation of MILAST should be considered.
Contraindications
MILAST is contraindicated:
In patients with known hypersensitivity to the active substance or to any of the excipients.
During pregnancy and in nursing women.
Special Warnings and Precautions for Use
Weight Decrease In some patients treatment with MILAST has been associated with weight decrease.
Patients treated with MILAST should have their weight monitored regularly. If unexplained or clinically significant weight loss occurs, weight loss should be evaluated, and discontinuation of MILAST should be considered (see section adverse effects (Undesirable effects)).
Depression Treatment with MILAST is associated with an increase in occurrences of depression (see section Adverse effects (Undesirable effects)).
Before using MILAST in patients with a history of depression and/or suicidal thoughts or behavior, prescribers should carefully weigh the risks and benefits of treatment with MILAST in such patients. Patients, their caregivers, and families should be advised of the need to be alert for the emergence or worsening of depression, suicidal thoughts or other mood changes, and if such changes occur to contact their healthcare provider. Prescribers should carefully evaluate the risks and benefits of continuing treatment with MILAST if such events occur. Diarrhea, Nausea and Vomiting There have been post‐marketing reports of severe diarrhea, nausea, and vomiting associated with the use of MILAST. Most events occurred within the first few weeks of treatment. In some cases, patients were hospitalized.
Patients 65 years of age or older and patients taking medications that can lead to volume depletion or hypotension may be at a higher risk of complications. Patients who reduced dosage or discontinued MILAST generally improved quickly.
Consider MILAST dose reduction or suspension if patients develop severe diarrhea, nausea or vomiting. Use in Patients with Lactose Intolerance MILAST tablets contain lactose.
Patients with rare hereditary problems of galactose Intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicinal product. Use in Renal Impairment Assessment of renal function is recommended prior to initiation of MILAST.
Use in the elderly. MILAST was studied in young and elderly healthy subjects. The exposure in elderly subjects (65 to 85 years of age) is about 13% higher in AUC and about 6% higher in Cmax for apremilast than that in young subjects (18 to 55 years of age). No overall differences were observed in the safety or efficacy profile of elderly patients’ ≥ 65 years of age and younger adult patients < 65 years of age in the clinical studies. No dosage adjustment is necessary for elderly patients. Pediatric Use The safety and effectiveness of MILAST has not been established in patients under the age of 18 years.
Effects on Laboratory Tests No data available.
Interaction MILAST has not been studied in combination with cyclosporine or biologic therapies.
Effect of MILAST on Other Medicinal Products
There was no pharmacokinetic drug-drug interaction between MILAST and methotrexate. MILAST can be co-administered with methotrexate. There was no pharmacokinetic drug-drug interaction between MILAST and oral contraceptives containing ethinyl estradiol and norgestimate. MILAST can be taken with oral contraceptives without clinically relevant drug-drug interaction.
In vitro, apremilast is not an inhibitor or inducer of cytochrome P450 enzymes. Hence, apremilast co-administered with substrates of CYP enzymes is unlikely to affect the clearance and exposure of drugs that are metabolized by CYP enzymes.
In vitro, apremilast is a substrate, and a weak inhibitor of P-glycoprotein (IC > 50 µM). In vitro, apremilast has little to no inhibitory effect (IC50 > 10µM) on Organic Anion Transporter (OAT) 1 and OAT3, Organic Cation Transporter (OCT) 2, Organic Anion Transporting Polypeptide (OATP) 1B1 and OATP1B3, or breast cancer resistance protein (BCRP) and is not a substrate for these transporters. Hence, clinically relevant drug-drug interactions are unlikely when apremilast is co-administered with drugs that are substrates or inhibitors of these transporters.
Effect of Other Medicinal Products on MILAST Co-administration of MILAST with multiple doses of rifampicin resulted in a decrease in apremilast area-under-the concentration time curve (AUC) and maximum serum concentration (Cmax) by approximately 72% and 43%, respectively. Apremilast exposure is decreased when administered concomitantly with strong inducers of CYP3A4 (e.g. rifampicin, phenobarbitone, carbamazepine, phenytoin and St. John’s Wort) and may result in reduced clinical response. Ketoconazole co-administration increased mean apremilast AUC 0-∞ and Cmax by approximately 36% and by 5%, respectively, which is not clinically meaningful. MILAST can be co-administered with a potent CYP3A4 inhibitor like ketoconazole.
Fertility, Pregnancy and Lactation Effects on Fertility No fertility data is available in humans. In a male mouse fertility study, apremilast at oral dosages of 1, 10, 25, and 50 mg/kg/day produced no effects on male fertility; the no observed adverse effect level (NOAEL) for male fertility was greater than 50 mg/kg/day (3-fold clinical exposure). In a combined female mouse fertility and embryo-fetal developmental toxicity study with oral dosages of 10, 20, 40, and 80 mg/kg/day, a prolongation of oestrous cycles and increased time to mating were observed at 20 mg/kg/day and above; despite this, all mice mated and pregnancy rates were unaffected. The no observed effect level (NOEL) for female fertility was 10 mg/kg/day (1.0-fold clinical exposure).
Use in Pregnancy (Pregnancy Category B3)
There are no adequate and well controlled studies of MILAST in pregnant women. MILAST is contraindicated in pregnant women, and should not be used in women attempting to become pregnant.
Apremilast was not teratogenic in mice or monkeys. Other effects of apremilast on pregnancy included embryofoetal loss in mice and monkeys, and reduced fetal weights and delayed ossification in mice at doses higher than the currently recommended highest human dose. No such effects were observed when exposure in animals was at 1.3-fold the clinical exposure.
In a combined female mouse fertility and embryofoetal developmental toxicity study, the maternal and developmental NOEL observed was 10 mg/kg/day (1.3-fold clinical exposure). No treatment-related developmental malformations were observed up to the highest dosage of 80 mg/kg/day (4.0-fold clinical exposure).
In a monkey embryofoetal developmental toxicity study, oral dosages of 20, 50, 200, and 1,000 mg/kg/day resulted in a dose-related increase in prenatal loss (abortions) at dosages of 50 mg/kg/day and above; no test article-related effect in prenatal loss was observed at 20 mg/kg/day (1.4-fold clinical exposure). No treatment-related fetal developmental effects or malformations were observed in the monkey up to the highest dosage of 1,000 mg/kg/day in the study (3.5-fold clinical exposure).
In a pre- and postnatal study, in which apremilast was administered orally to pregnant female mice, clinical signs of maternal toxicity associated with delivering pups were observed in one mouse at each of 80 and 300 mg/kg/day. Increased pre- and postnatal pup mortality and reduced pup body weights during the first week of lactation were observed at ≥ 80 mg/kg/day (≥ 4.0-fold clinical exposure). The NOEL in the mouse for maternal toxicity and F1 generation was 10 mg/kg/day (1.3-fold clinical AUC).
Use in Lactation Apremilast was detected in milk of lactating mice. It is not known whether apremilast or its metabolites are excreted in human milk. Therefore, the use of MILAST is contraindicated in mothers who are breast-feeding.
Effects on Ability to Drive and Use Machines No studies on the effects on the ability to drive and use of machines have been performed.
Adverse Effects (Undesirable Effects)
Summary of Safety Profile Apremilast was evaluated in 4 multi-center, randomized, double-blind, placebo-controlled trials (Studies PALACE 1, PALACE 2, PALACE 3 and PALACE 4) of similar design in adult patients with active psoriatic arthritis. Across the 4 studies, there were 1,945 patients who received at least one dose of Apremilast 20 mg twice daily or Apremilast 30 mg twice daily.
Apremilast was evaluated in 2 multi-center, randomized, double-blind, placebo-controlled trials (Studies ESTEEM 1 and ESTEEM 2) of similar design in adult patients with moderate to severe plaque psoriasis. Across the two studies, 1,184 psoriasis patients were exposed to Apremilast 30 mg twice daily.
Hypersensitivity reactions were observed infrequently in clinical studies with MILAST.
Frequencies are defined as:
very common (≥1/10), common (≥1/100 to <1/10); and uncommon (≥1/1,000 to <1/100) or rare (≥ 1/10,000 to < 1/1,000).
Gastrointestinal Disorders
Very Common
Diarrhea
Nausea
Common
Vomiting
Frequent bowel movements
Abdominal pain upper
Gastro esophageal reflux disease
Dyspepsia
General Disorders and Administrative Site Conditions
Common:
Fatigue Immune System Disorders
Uncommon:
Hypersensitivity Infections and Infestations
Common: Bronchitis Upper Respiratory Tract Infection Nasopharyngitis
Investigational Adverse Effects:
Uncommon: Weight decrease Metabolism and Nutrition Disorders
Common: Decreased appetite Musculoskeletal and Connective Tissue
Common: Back Pain
Nervous System Disorders
Common: Migraine, Tension headache, Headache
Psychiatric Disorders
Common: Insomnia Respiratory,
Thoracic, and Mediastinal Disorders
Common: Cough Skin and Subcutaneous Tissue Disorders
Uncommon: Rash The most commonly reported adverse reactions in Phase 3 (Studies PALACE 1, PALACE 2, PALACE 3, PALACE 4 and ESTEEM 1 and ESTEEM 2) clinical studies have been gastrointestinal (GI) disorders including diarrhea (15.7%) and nausea (13.9%). These GI adverse reactions were mostly mild to moderate in severity, with 0.3% of patients reporting severe diarrhea and 0.3% of patients reporting severe nausea.
These adverse reactions generally occurred within the first 2 weeks of treatment and usually resolved within 4 weeks. The other most commonly reported adverse reactions included upper respiratory tract infections (8.4%), headache (7.9%), and tension headache (7.2%).
Overall, most adverse reactions were considered to be mild or moderate in severity. The most common adverse reactions leading to discontinuation during the first 16 weeks of treatment were diarrhea (1.7%), and nausea (1.5%).
Description of Selected Adverse Reactions:
Weight Decrease Patient weight was measured routinely in clinical studies. The mean observed weight loss in patients treated for up to 52 weeks with apremilast was 1.99 kg. A total of 14.3% of patients receiving apremilast had observed weight loss between 5-10% while 5.7% of the patients receiving apremilast had observed weight loss greater than 10%. None of these patients had overt clinical consequences resulting from weight loss. A total of 0.1% of patients treated with apremilast discontinued due to adverse reaction of weight decreased. Weight decreases of greater than 5% of baseline body weight were observed more frequently in women than in men.
Depression
Psoriatic Arthritis: During the 0 to 16-week placebo-controlled period of the 3 controlled clinical trials, 0.9% (18/1,945) of subjects treated with MILAST reported depression or depressed mood compared to 0.7% (5/671) treated with placebo. During the clinical trials, 0.1% (4/1,945) of subjects treated with MILAST discontinued treatment due to depression or depressed mood compared with none in placebo treated subjects (0/671). Depression was reported as serious in 0.2% (3/1,945) of subjects exposed to MILAST, compared to none in placebo-treated subjects (0/671). Instances of suicidal ideation and behavior have been observed in 0.2% (3/1,945) of subjects while receiving
MILAST, compared to none in placebo treated subjects (0/671). In the clinical trials, 2 subjects who received placebo committed suicide compared to none in MILAST treated subjects. Psoriasis: During the 0 to 16-week placebo-controlled period of the 2 controlled clinical trials, 1.2% (14/1,184) of subjects treated with MILAST reported depression compared to 0.5% (2/418) treated with placebo. During the clinical trials, 0.1% (1/1,184) of subjects treated with MILAST discontinued treatment due to depression compared with none in placebo-treated subjects (0/418). Depression was reported as serious in 0.1% (1/1,184) of subjects exposed to MILAST, compared to none in placebo-treated subjects (0/418). Instances of suicidal behavior have been observed in 0.1% (1/1,184) of subjects while receiving MILAST, compared to 0.2% (1/418) in placebo-treated subjects. In the clinical trials, one subject treated with MILAST attempted suicide while one who received placebo committed suicide. Safety in elderly patients
No overall differences were observed in the safety profile of elderly patients’ ≥ 65 years of age and younger adult patients < 65 years of age in the clinical studies.
Reporting of Suspected Adverse Effects Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Overdose MILAST was studied in healthy subjects at a maximum total daily dose of 100 mg (given as 50 mg twice daily) for 4.5 days without evidence of dose limiting toxicities. Patients should be managed by symptomatic and supportive care should there be an overdose.
PHARMACOLOGICAL PROPERTIES
Pharmacotherapeutic Group: Selective Immunosuppressant.
Pharmacodynamics properties
Mechanism of Action
Apremilast, an oral small-molecule inhibitor of phosphodiesterase 4 (PDE4), works intracellularly to modulate a network of pro-inflammatory and anti-inflammatory mediators. PDE4 is a cyclic adenosine monophosphate (cAMP)-specific PDE and the dominant PDE in inflammatory cells. PDE4 inhibition elevates intracellular cAMP levels, which in turn downregulates the inflammatory response by modulating the expression of TNF-α, IL-23, IL-17 and other inflammatory cytokines. Cyclic AMP also modulates levels of anti-inflammatory cytokines such as IL-10. These pro- and anti-inflammatory mediators have been implicated in PsA and PSOR.
Clinical Pharmacodynamics In clinical studies in patients with psoriatic arthritis, apremilast significantly modulated, but did not fully inhibit, plasma protein levels of IL-1α, IL-6, IL-8, MCP-1, MIP-1β, MMP-3, and TNFα. After 40 weeks of treatment with apremilast, there was a decrease in plasma protein levels of IL-17 and IL-23, and an increase in IL-10. In clinical trials in patients with psoriasis, apremilast decreased lesional skin epidermal thickness, inflammatory cell infiltration, and expression of
pro-inflammatory genes, including inducible nitric oxide synthase (iNOS), IL-12/IL-23p40, IL17A, IL-22 and IL-8. Cardiac Electrophysiology Apremilast administered at doses of up to 50 mg twice daily did not prolong the QT interval in healthy subjects.
Pharmacokinetic Properties
Absorption
Apremilast is well absorbed with an absolute oral bioavailability of approximately 73%, with peak plasma concentrations (Cmax) occurring at a median time (tmax) of approximately 2.5 hours. Apremilast pharmacokinetics is linear, with a dose-proportional increase in systemic exposure in the dose range of 10 to 100 mg daily. Accumulation is minimal when apremilast is administered once daily and approximately 53% in healthy subjects and 68% in patients with psoriasis when administered twice daily. Co-administration with food does not alter the bioavailability; therefore, apremilast can be administered with or without food.
Distribution
Human plasma protein binding of apremilast is approximately 68%. Mean apparent volume of distribution (Vd) is 87 L indicative of extra vascular distribution.
Metabolism
Apremilast is extensively metabolized by both CYP and non-CYP mediated pathways including oxidation, hydrolysis, and conjugation, suggesting inhibition of a single clearance pathway is not likely to cause a marked drug-drug interaction. Oxidative metabolism of apremilast is primarily mediated by CYP3A4, with minor contributions from CYP1A2 and CYP2A6. Apremilast is the major circulating component following oral administration. Apremilast undergoes extensive metabolism with only 3% and 7% of the administered drug recovered in urine and faces, respectively. The major circulating metabolite, M12, is the glucuronide conjugate of Odemethylated apremilast which is inactive.
Excretion
The plasma clearance of apremilast is on average about 10 L/hr. in healthy subjects, with a terminal elimination half-life of approximately 9 hours. There is approximately 30% reduction in apremilast clearance observed in female subjects compared to male subjects. No dose adjustment is necessary for female patients. Following oral administration of radiolabelled apremilast, about 58% and 39% of the radioactivity is recovered in urine and faces, respectively, with about 3% and 7% of the radioactive dose recovered as apremilast in urine and faces, respectively. Renal Impairment No formal studies have been conducted in subjects with mild to moderately impaired renal function. In 8 subjects with severe renal impairment administered a single dose of 30 mg apremilast, the AUC and Cmax of apremilast increased by approximately 89% and 42%, respectively. See section 4.2 Dose and method of administration for dose adjustments for patients with severe renal impairment. Hepatic Impairment
The pharmacokinetics of apremilast and its major metabolite M12 is not affected by moderate or severe hepatic impairment. No dosage adjustment is necessary for patients with hepatic impairment.
Preclinical Safety Data
Genotoxicity Apremilast is not genotoxic.
Apremilast did not induce mutations in an Ames assay or chromosome aberrations in cultured human peripheral blood lymphocytes in the presence or absence of metabolic activation. Apremilast was not clastogenic in an in vivo mouse micronucleus assay at doses up to 2,000 mg/kg/day.
Carcinogenicity
Carcinogenicity studies showed no evidence of treatment-related tumors following oral treatment with apremilast at plasma exposure levels (AUC) that were 7 to 10-fold (mice) and 0.1 to 1-fold (rats) than anticipated clinically.
Special Precautions for Storage
Store not exceed 30°C.