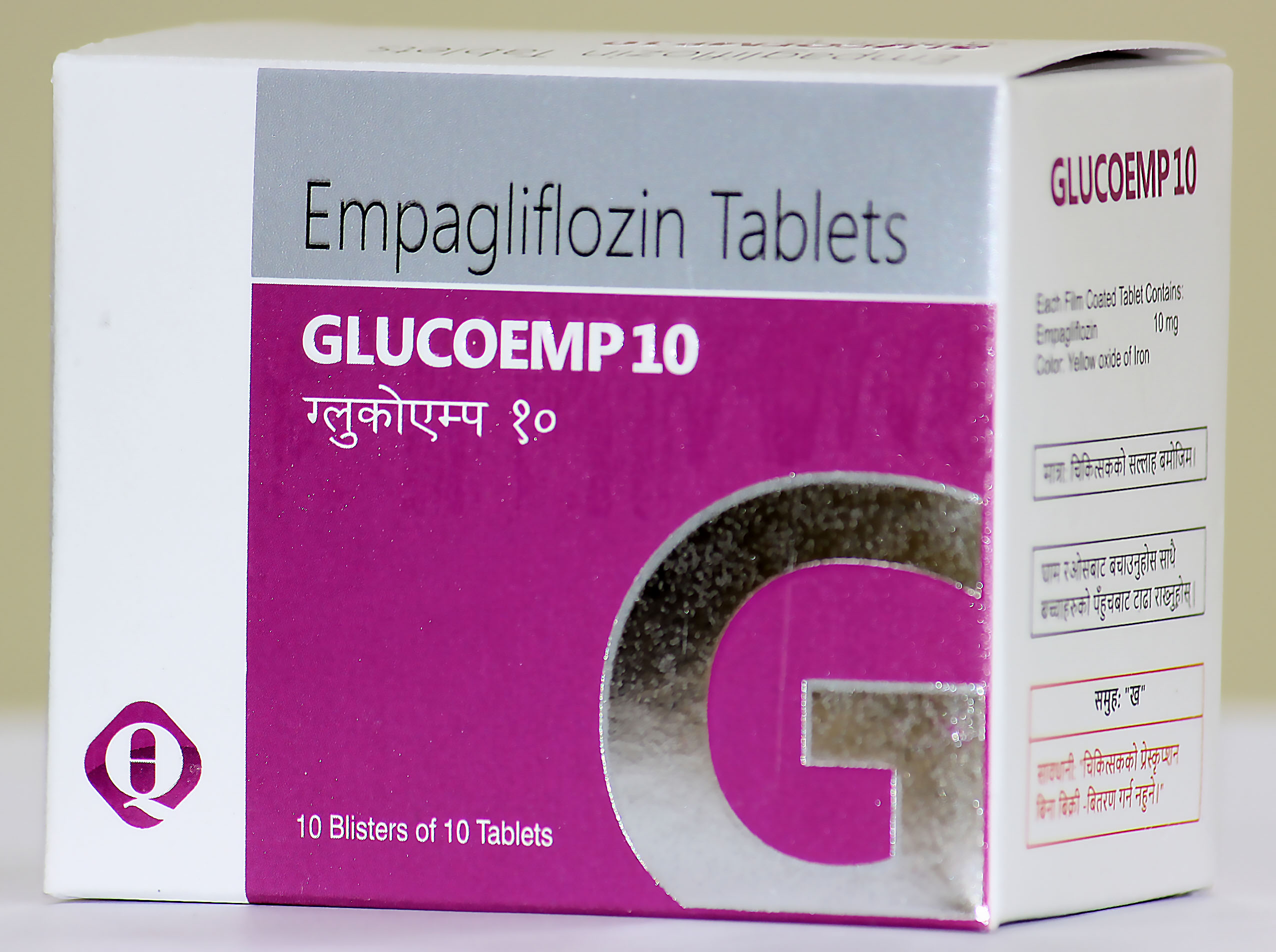
It is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and
- As monotherapy when Metformin is considered inappropriate due to intolerance
- In addition to other medicinal products for the treatment of diabetes.