
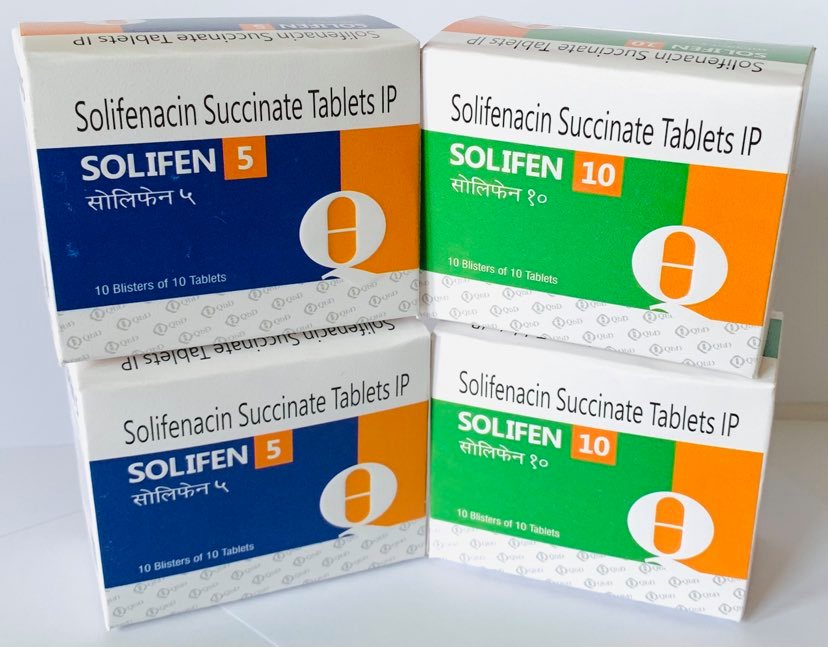
The active ingredient in SOLIFEN is Solifinacin Succinate, a competitive muscarinic receptor antagonist.
Each tablet contains Solifinacin Succinate 5mg/10mg.
Pack size: 10*10 Blisters.
Pharmacodynamics Properties
Mechanism of Action
Solifenacin is a competitive muscarinic receptor antagonist. Muscarinic receptors play an important role in several major cholinergic mediated functions, including contractions of urinary bladder smooth muscle and stimulation of the salivary secretion.
After intake of Solifen tablets, maximum Solifenacin plasma concentrations (Cmax) are reached after 3 to 8 hours and at steady state ranged from 32.3 to 69.9 ng/ml for 5 and 10 mg Solifen tablets, respectively. The tmax is independent of the dose. The Cmax and area under the curve (AUC) increase in proportion to the dose between 5 to 40 mg. Absolute bioavailability is approximately 90%. Food intake does not affect the Cmax and AUC of Solifen.
The apparent volume of distribution of solifenacin following intravenous administration is about 600 L. Solifenacin is to a great extent (approximately 98%) bound to plasma proteins, primarily α1-acid glycoprotein.
Solifenacin is extensively metabolized in the liver. The primary pathway for elimination is by way of CYP3A4; however, alternate metabolic pathways exist. The primary metabolic routes of Solifenacin are through N-oxidation of the quinuclidin ring and 4R-hydroxylation of tetra hydro isoquinoline ring. One pharmacologically active metabolite (4R-hydroxy solifenacin), occurring at low concentrations and unlikely to contribute significantly to clinical activity, and three pharmacologically inactive metabolites (N-glucuronide and the Noxide and 4R-hydroxy-N-oxide of Solifenacin) have been found in human plasma after oraldosing.
After a single administration of 10 mg [14C-labelled]-Solifenacin, about 70% of the radioactivity was detected in urine and 23% in faeces over 26 days. In urine, approximately 11% of the radioactivity is recovered as unchanged active substance; about 18% as the Noxide metabolite, 9% as the 4R-hydroxy-N-oxide metabolite and 8% as the 4R-hydroxy metabolite (active metabolite). The systemic clearance of Solifenacin is about 9.5 L/h. The elimination half-life of Solifenacin following chronic dosing is approximately 45 – 68 hours.
Inducers or inhibitors of CYP3A4 may alter Solifen pharmacokinetics. Simultaneous administration of Ketoconazole (200 mg/day), a potent CYP3A4 inhibitor, resulted in a twofold increase of the AUC of Solifenacin, while Ketoconazole at a dose of 400 mg/day resulted in a three-fold increase of the AUC of Solifenacin. Therefore, the maximum dose of Solifen should be restricted to 5 mg, when used simultaneously with Ketoconazole or therapeutic doses of other potent CYP3A4 inhibitors (e.g. Ritonavir, Nelfinavir, Itraconazole, Cyclosporin, Macrolide antibiotics).
Intake of Solifen showed no pharmacokinetic interaction of Solifenacin on combined oral contraceptives (ethinyl oestradiol/levonorgestrel).
Intake of Solifen did not alter the pharmacokinetics of R-warfarin or S-warfarin or their effect on prothrombin time.
Intake of Solifen showed no effect on the pharmacokinetics of Digoxin.
Solifen is indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency or increased urinary frequency
The recommended dose is 5 mg Solifen is once daily. If needed, the dose may be increased to a maximum of 10 mg Solifen once daily.
Safety and effectiveness in children have not yet been established. Therefore, Solifen should not be used in children.
Solifen should be taken orally and should be swallowed whole with liquids. It can be taken with or without food.
Patients with renal impairment: No dose adjustment is necessary for patients with mild to moderate renal impairment (creatinine clearance > 30 ml/min). Patients with severe renal impairment (creatinine clearance ≤ 30 ml/min) should be treated with caution and receive no more than 5 mg once daily.
No dose adjustment is necessary for patients with mild hepatic impairment. Patients with moderate hepatic impairment (Child-Pugh B) should be treated with caution and receive no more than 5 mg once daily. Solifen is not recommended for patients with severe hepatic impairment (Child-Pugh C).
The maximum dose of Solifen should be limited to 5 mg when treated simultaneously with Ketoconazole or therapeutic doses of other potent CYP3A4-inhibitors e.g. Ritonavir, Nelfinavir, Itraconazole, Cyclosporin, Macrolide antibiotics.
flatulence, gastro-esophageal reflux diseases, throat irritation, eructation, dry throat
cystitis
somnolence, dysgeusia, syncope
thirst, suprapubic pain, chest tightness
difficulty in micturition, bladder pain, micturition urgency respiratory, thoracic and
nasal dryness
abnormal liver function tests (AST, ALT, GGT), electrocardiogram QT prolonged
peripheral swelling
dry skin
hot flushes
Solifen should be used with caution in patients with
If angioedema occurs, Solifen should be discontinued and appropriate therapy and/or measures should be taken.
In patients who develop anaphylactic reactions, Solifen should be discontinued and appropriate therapy and/or measures taken.
Solifen should be used with caution in patients with reduced renal function. Solifen should be used with caution in patients with severe renal impairment (creatinine clearance < 30 ml/min), and doses should not exceed 5 mg for these patients.
Doses of Solifen greater than 5 mg are not recommended in patients with moderate hepatic impairment (Child-Pugh B). Solifen is not recommended for patients with severe hepatic impairment (Child-Pugh C).
Co administration of drugs known to prolong the QT interval) and relevant pre-existing cardiac diseases (i.e. myocardial ischemia, arrhythmia, congestive heart failure). Appropriate investigations (e.g. ECG) should be considered in patients with risk factors for
Solifen had no effect on reproductive function, fertility or early embryonic development after oral treatment of male and female mice, which resulted in 13 times exposure at the maximum recommended human dose (MRHD).
Solifen (and/or its metabolites) has been shown to cross the placenta in pregnant mice. Solifen should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is expected that Solifenacin is excreted in human milk and Solifen should not be administered during breast-feeding
Solifen is contraindicated in patients with